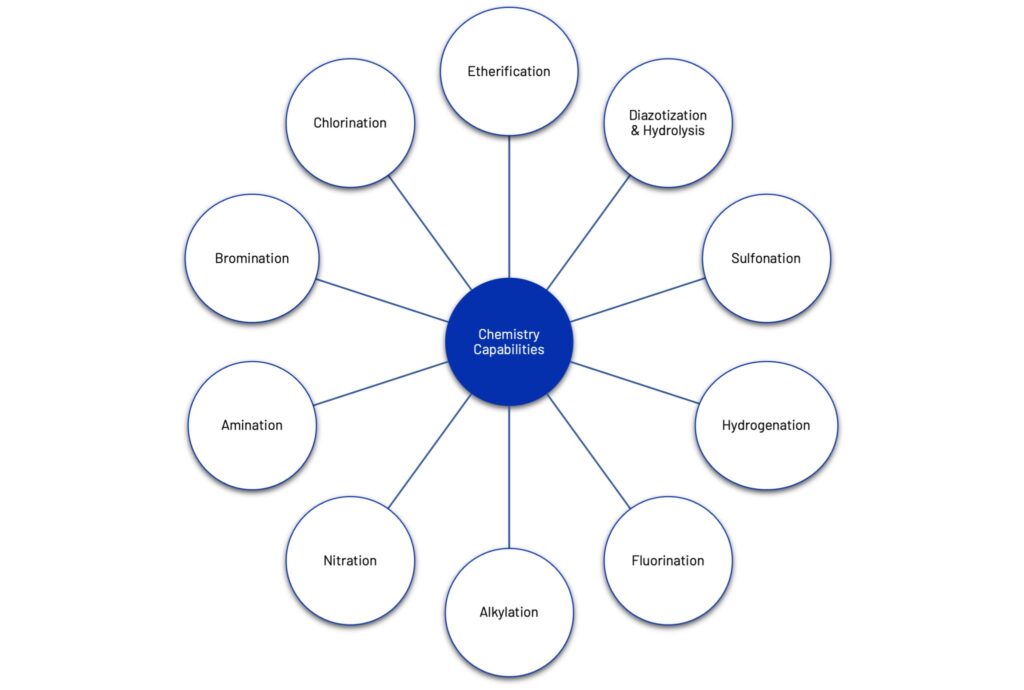
 As many companies look to diversify their Asian supply base – Austin can offer a cost efficient, full service CDMO in India that offers end-to-end support to pharmaceutical companies from research and development to commercial production. Having helped clients bring their programs from the IND stage to commercialization, they offer core competencies including non-GMP and GMP intermediate manufacturing, clinical-stage API manufacturing, enzymatic reactions, batch mode and continuous flow chemical process R&D they are ideally suited to assist needs of both virtual and large pharma. For more information contact Austin Chemical.
As many companies look to diversify their Asian supply base – Austin can offer a cost efficient, full service CDMO in India that offers end-to-end support to pharmaceutical companies from research and development to commercial production. Having helped clients bring their programs from the IND stage to commercialization, they offer core competencies including non-GMP and GMP intermediate manufacturing, clinical-stage API manufacturing, enzymatic reactions, batch mode and continuous flow chemical process R&D they are ideally suited to assist needs of both virtual and large pharma. For more information contact Austin Chemical.
Cost Efficient, Full Service CDMO in India
You are here:
- Home
- Austin News
- Cost Efficient, Full Service CDMO…
